Study Management & Monitoring for Virtual & Remote
Clinical Trials
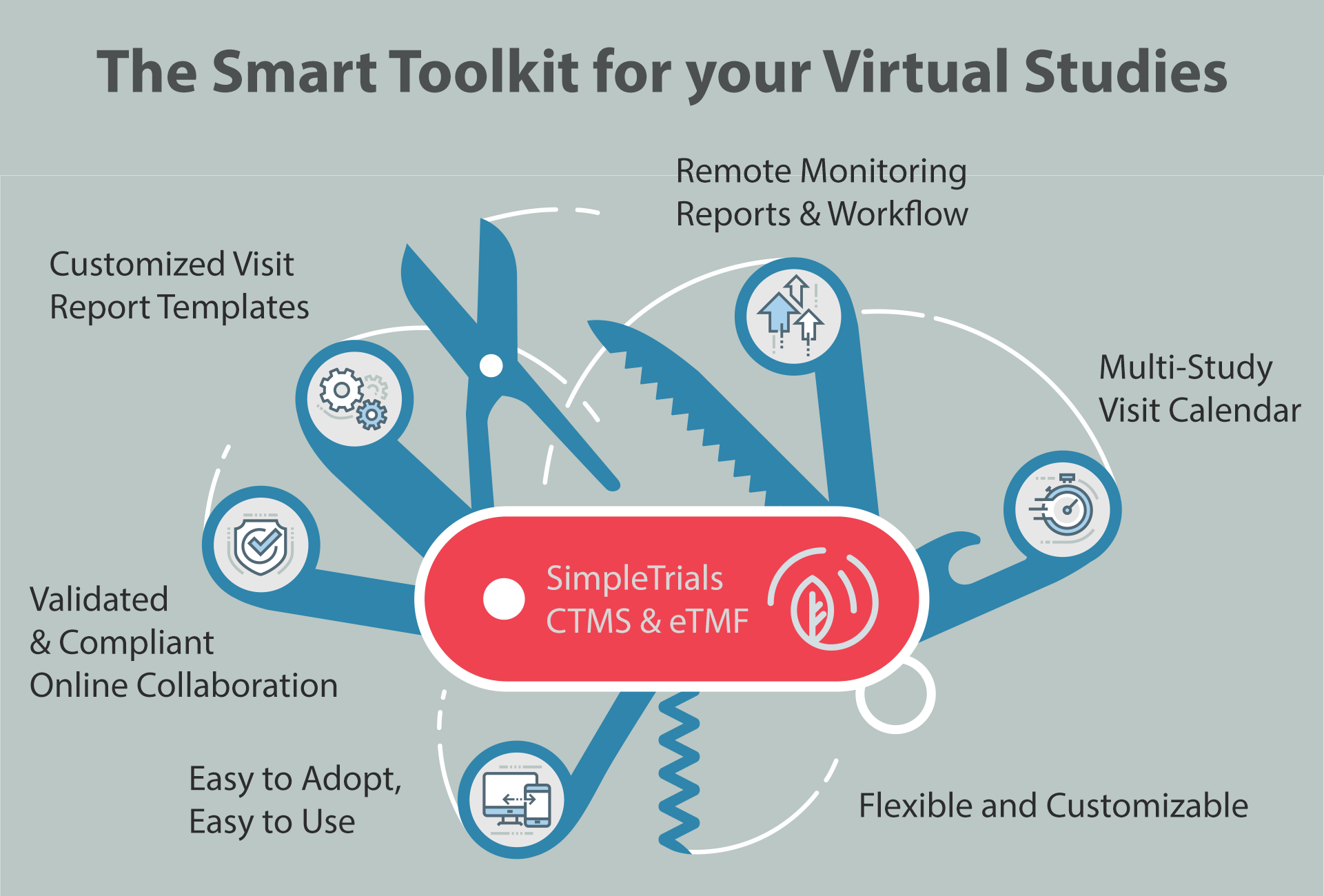
SimpleTrials is the Easy-To-Use, Collaborative, Flexible CTMS & eTMF Solution
The rise of virtual, remote and decentralized clinical trial models are disrupting the traditional approach to study management. SimpleTrials CTMS, with integrated eTMF, provides a secure, web-based, study management suite for your remote clinical team.
Designed by clinical trial experts, SimpleTrials bridges every part of your trial management to help make them simple, efficient and compliant.
Remote Monitoring Visit Reports
- Within one study, manage both remote and on site visits.
- The multi-study calendar provides quick visit oversight.
- Full eVisit Report workflow, including authoring, revision, and approval.
Custom Visit Report Templates
- Easy to use report template builder.
- Templates are fully customizable for remote and on-site visits.
- Action Items and Protocol Deviations roll-up from all visit reports to an aggregated view for quick & easy oversight.
Study Management Oversight
- Real-time data for remote decision making.
- Dashboards provide visualization of key metrics, including data points for risk based monitoring.
- Custom CTMS Reports put a wealth of data at your fingertips.
Simple, Well Organized eTMF
- Build your folder structure using the DIA reference model or a simplified custom model.
- Collect essential documents via upload or email attachment.
- Track missing documents in app and via reports.
- Perform QC and get alerted for expired documents.
Secure and Compliant
- Online collaboration for your local and global users, in a fully validated system.
- 21 CFR Part 11 compliant, with electronic signatures and full audit trail.
- User account controls with multi-factor authentication avilable.
Example of Charts from the SimpleTrials Study Dashboard